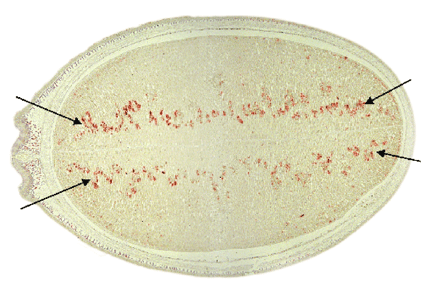
The abundant soybean seed proteins are the globulins that are classified into 7S (ß-conglycinin) and 11S (glycinin). These two groups of proteins account for about 70% of the total soybean seed storage proteins. Because of their abundance, these proteins are mainly responsible for the nutritional quality of soybean seed proteins. However, the overall content of methionine and cysteine in soybean seed proteins is about half of the egg protein. This deficiency creates a problem in animal feeds especially in poultry and swine. To meet the deficiency, the animal industry supplements the soybean-based rations with synthetic methionine, at an estimated cost of 100 million dollars annually. Currently we are employing two molecular approaches to improve the concentration of limiting amino acids in soybean seed proteins. One approach involves the expression of methionine-rich delta-zeins under the control of the ß-conglycinin alpha’-subunit promoter in transgenic soybean plants.
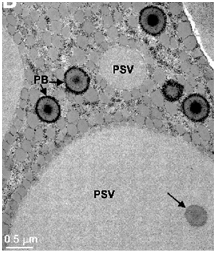
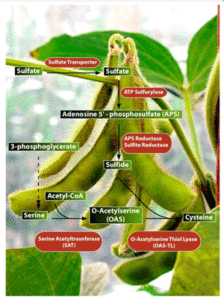
In another approach we are identifying and manipulating the enzymes involved in sulfate uptake and assimilation in soybean. In spite of the critical importance of sulfur metabolism in soybean, very little is known about this pathway. The cysteine biosynthetic pathway is responsible for the fixation of sulfate into L-cysteine. Sulfate is absorbed by the roots and transported to other plant tissues, a process that is mediated by the sulfate transporters. The reduction of sulfate to sulfide follows, which in turn enters the cysteine synthesis pathway to form cysteine, reactions catalyzed by serine acetyltrasnferase (SAT; EC 2.3.1.30) and cysteine synthase or O-acetylserine thiol lyase (OAS-TL; EC 4.2.99.8) (Leustek and Saito 1999). SAT catalyzes the formation of O-acetylserine (OAS) from serine and acetyl-CoA. OAS will then combine with sulfide to form cysteine, reaction catalyzed by OAS-TL. Currently, we are employing biochemical, structural and molecular biological approaches to manipulate the expression of key sulfur assimilatory enzymes with goal of increasing seed cysteine and methionine levels.
Another area of our research is focused on biological nitrogen fixation using the model symbiosis between soybeans and Sinorhizobium fredii USDA257. Efficient nitrogen fixation greatly enhances the yield of protein-rich seeds. Sinorhizobium fredii USDA257 nodulates primitive but not agronomically improved soybean cultivars. Our laboratory is currently focusing on the cultivar-specificity genes of S. fredii. What do they encode? What regulates their expression? And how are the responses are made host specific? Answers to such questions will enable us to rationally manage and enhance the process of biological nitrogen fixation and improve the overall soybean protein quality.


Nodulation of Soybean by Sinorhizobium fredii is Cultivar Specific
